MediaPharma-Aegirbio R&D Agreement
Aegirbio AB has today signed a research & development agreement with MediaPharma Srl, an Italian biotech company focusing on novel drugs for the cure of cancer, and through this agreement Aegirbio intends to develop these drugs together with MediaPharma, in oncology and other areas.
The unique characteristic of the cooperation is that Aegirbio’s Veritope technology is combined with MediaPharma’s portfolio already in the development phase. Therapeutic Drug Monitoring, where diagnostics and drugs are developed in tandem will improve the precision of the treatment even further. In addition, MediaPharma will be able to move through the clinical phases of drug development faster and more reliably.
This represents a major stride, where Aegirbio opens up a novel business area centred on “Companion Diagnostics”; we are convinced that pharmaceutical companies will adopt such a concurrent focus on both the monitoring and the drug as a standard practice of drug development.
“MediaPharma has decided to join forces with Aegirbio by using its veritope technology coupled with our portfolio, including monoclonal antibodies, bispecifics and Antibody-Drug Conjugates (ADC)s, during the development phase. We believe that the addition of Therapeutic Drug Monitoring will enhance the treatment accuracy for patients,” says Stefano Iacobelli, CEO of MediaPharma Srl .
“It is exciting to be given the opportunity to utilize our Veritope technology together with a drug developing company. We believe ensuring at an early stage that therapies are individualized to be the future of drug development. Furthermore, it is our conviction that this will accelerate and secure the clinical trials. We take the fact that a successful team such as MediaPharma has chosen to cooperate with us as a further confirmation of our technology’s prowess,” says Martin Linde, CEO of Aegirbio AB.
About Aegirbio
Aegirbio is a Swedish diagnostics company offering tests to monitor and optimize the dosing of biological drugs by means of a unique, patented technology platform. Biological therapies is the fastest growing segment of the pharmaceutical industry; a quarter of all drugs are projected to be biological in 2020. At the same time, drug concentrations vary tremendously (up to 100 times) in patients that receive biological drugs in standard doses.
The result of this one size fits all-approach is that patients with low drug concentrations do not respond to treatment, while excessive drug concentrations increase the risk of adverse effects in others. The uncertainty surrounding dosing results in overdosing or underdosing in about 55 percent of the cases, which causes unnecessary costs and suboptimal clinical outcomes.
The Company’s tests for optimal dosing of biological drugs will be focused on neurological disorders, autoimmune diseases and cancer. In the first quarter of 2020, Aegirbio initiated sales in the U.S. of the MoNATor test for the drug Tysabri, which is used for treatment of the neurological disorder Multiple Sclerosis (MS). Aegirbio’s goal is to launch a total of four tests before 2023. Diagnostics will be offered through laboratory testing as well as in the form of a P.O.C. (Point of Care) test for use in hospitals and health centres. The plan further includes P.O.N. (Point of Need) tests for use at home.
For more information, please visit Aegirbio’s website, www.aegirbio.com
Certified adviser for the company is Eminova Fondkommission AB | +468-684 211 00 | info@eminova.se
About MediaPharma Srl
MediaPharma Srl, located in Rome and Chieti, Italy, is a drug discovery and development biotech company active in immuno-oncology and focusing on research and development of monoclonal antibodies, Antibody-Drug Conjugates (ADC)s and therapeutic proteins for treatment of cancer and other severe diseases with high medical need. Inherently innovative,
MediaPharma focuses specially on those cancers which have become resistant to previous drug regimens. At MediaPharma, world-class researchers, biologists, medical oncologists and entrepreneurs share the same values and have been working together for years to optimize patient’s treatment.
MediaPharma Srl
Via Colonnetta, 50/A
66000 Chieti
Italy
Pr. Stefano Iacobelli, CEO
Phone: +39 0871 563 273
Fax: +39 0871 563273
www.mediapharma.it
MP-1959-ADC
Humanized Anti-Gal-3 BP Antibody-Drug Conjugate
MP-1959 is the humanized version of the murine monoclonal antibody SP-2 which recognizes human Gal-3 BP (also known as LGALS3BP, 90K or Mac-2 BP), a glycoprotein secreted in large amounts by the majority of tumor cells, which plays an important role in cell-cell and cell-extracellular matrix adhesion, angiogenesis and tumor invasion . MediaPharma’s scientists have generated a non-internalizing anti-Gal-3 BP Antibody-Drug Conjugate MP-1959.ADC. It consists of a humanized, engineered anti-Gal-3 BP monoclonal antibody (1959-sss) conjugated to highly potent maytansinoids (DM3 & DM4) via a disulfide bond to residual cysteine of the antibody’s light chain. Administration of LGS-ADC was effective in inducing a long lasting tumor shrinkage of tumor cell lines and patient-derived tumor xenografts.
Indications in Oncology: cancers overexpressing Gal-3 BP, including those arising from breast, prostrate, ovary, pancreas, lung, melanoma, glioblastoma and neuroblastoma.
MP-EV20-ADC
Humanized anti-HER3 Antibody-Drug Conjugate
MP-1959
Anti-Gal-3BP Antibody
MP-1959 is the humanized version of the murine monoclonal antibody SP-2 which was originally raised against human Gal-3 BP (also known as LGALS3BP, 90K or Mac-2 BP), a glycoprotein secreted in large amounts by the majority of cancer cells. Functionally, Gal-3BP plays an important role in cell-cell and cell-extracellular matrix adhesion, angiogenesis, tumor progression and metastasis.
MP-1959, given alone or in combination with other biological agents has shown significant therapeutic activity in several xenograft models, including breast cancer (triple negative), ovarian cancer, prostate cancer and head & neck cancer.
Personalized medicine
Despite extensive research, today all drugs used to cure cancer are applied blindly, without any knowledge of their efficacy as well as side effects they produce at the level of the single patient. The problem lies in the lack of suitable assays to select patients more likely to respond to a given anticancer therapy.
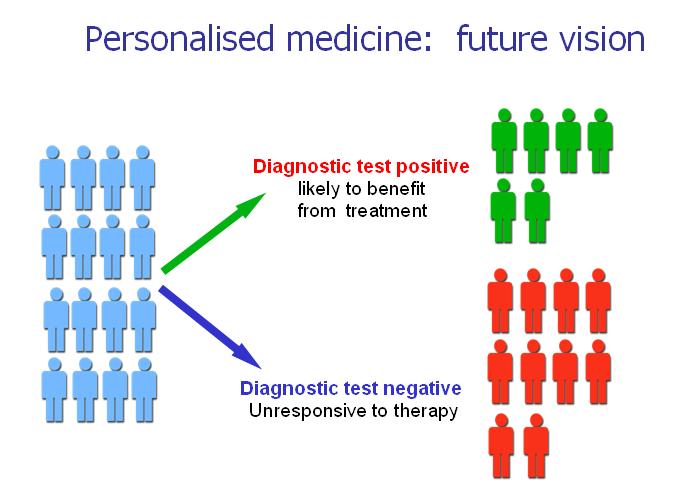
Of relevance has been the notion of “companion diagnostics”, whereby molecular assays that measure levels of proteins, genes, or specific mutations are used to provide a highly specific therapy for an individual’s cancer – by stratifying disease status, selecting the proper medication, and tailoring dosages to that patient’s specific needs. In other words, it is today possible to examine the impact of genetic/protein variation of the host and the tumor and predict drug responses and toxicities.
MediaPharma is focusing on personalized oncology by developing companion diagnostic tests along with the development of its antibody pipeline. The development of biomarkers that identify defined patient populations enables a more individualized approach to cancer treatment than previously possible and has the potential to reduce the cost of cancer care.
MediaPharma S.r.l.
A preclinical stage biotech company focusing on research and development of novel products for the diagnosis and treatment of cancer and other severe diseases with high unmet medical needs.









